Which of the Following Options Correctly Describe Daltons Atomic Theory
Dalton brought forward the theory and descriptions about an atom. According to the Postulates of Daltons atomic theory atoms are further indivisible and all matter is composed of smallest particles called atom.
What Is The Difference Between The Solid Atom In Dalton Atomic Theory And Thomson Atomic Model Quora
Option b is correct In Daltons atomic theory he didnt mention about the nucleus and its constituents of an atom.
. 4 All atoms of an element are identical. 3 Atoms can neither be created nor destroyed. The options available on paste menu are.
2 Atoms cannot be created or destroyed. All matter is made up of small particles called atoms. According to Johns Daltons modern theory of an atom.
2 All atoms of the same element are identical. The second part of the theory says all. Correct option is B Daltons atomic theory was given by John Dalton.
Dalton based his theory on the law of conservation of mass and the law of constant composition. 3 Atoms can neither be created nor destroyed. Which of the following options correctly describe Rutherfords experiment to discover the nature of the atom.
5 Chemical reactions cause atoms to be rearranged. Daltons atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Nuclear fission is a.
Daltons atomic theory stated that matter consists of tiny indivisible particles called atoms which combine in definite whole-number ratios to form compounds Thomson discovered that cathode rays emanate from the negative electrode. Keep source formatting A merge Formatting B Keep text only C Explanation. One key point in Daltons atomic theory was Chemical reactions involve reorganization of the atoms- changes the way they are bound together.
So the statement atoms can be divided into electrons protons and neutrons is not the postulate of Daltons atomic theory. Atoms are composed of electrons protons and neutrons. Daltons atomic theory.
Which of the following is not part of Daltons atomic theory. Select all that apply 1 Atoms have a massive positively charged center. 3 Atoms cannot be broken down into smaller pieces.
Compounds or chemical compounds. 2 Atoms cannot be created or destroyed. In the options under paste menu you will find.
Isotopes of the same element have different masses. Which of the following statements is. Daltons atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.
Put that idea with the common phrase you are what you eat. All matter is composed of atoms. View the full answer.
It involves the following postulates. Hey have the same outer electron configuration. A Matter is made up of invisible particles known as atoms.
Daltons atomic theory stated that matter consists of tiny indestructible particles called _____ which combine in definite ratios to form _____. 3 Atoms cannot be broken down into smaller pieces. Correct incorrect The following is the final score of.
Measurements of the angles between the screen metal foil and the source determined the scatter pattern of the particles. Describe the structure of the atom by explaining how Daltons atomic theory has changed due to scientific discoveries. A theory of chemical combination first stated by John Dalton in 1803.
This among other experimental evidence caused him to postulate that they consist of particles of the same charge. Dalton based his theory on the law of conservation of mass and the law of constant composition. 4 All atoms of an element are identical.
Paste menu is used after copyingcutting a textlink or image from one source and you want to move it to another source. Atoms are composed of electrons protons and neutrons. Option b is correct In Daltons atomic theory he didnt mention about the nucleus and its constituents of an atom.
Daltons atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. The first part of his theory states that all. Daltons atomic theory.
According to his theory. The four correct options are. Dalton based his theory on the law of conservation of mass and the law of constant composition.
Select all that apply. 5 Chemical reactions cause atoms to be rearranged. 1 Elements consist of indivisible small particles atoms.
Daltons atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. The answer for the following question is option C. The first part of his theory states that all matter is made of atoms which are indivisible.
C Atoms of different elements combine in fixed ratios to form compounds. Which of the following parts of John Daltons atomic theory if correct would have made it impossible for Marie Curies discovery that new elements can be produced when elements are bombarded with neutrons. The first part of his theory states that all matter is made of atoms which are indivisible.
The shortcut for pasting a copied item is Ctrl V. The first part of his theory states that all. B The properties of all the atoms of a given element are the same including mass.
Choose correct punctuation Wednesday is the picnic. Friday is the senior dance. Option C is not included in the John Daltons modern theory of an atom.
Which of the following options correctly describe Daltons atomic theory. A chemical reaction is a rearrangement of atoms. Which statements belong to daltons atomic theory.
Select all that apply. According to Dalton every matter is made up. Atoms of the same element are identical.
Different elements have different types of atom. It states atoms of different elements combine to form new compound but not new elements.

Solved Question 1 1 Point Dalton S Atomic Theory Was Based Chegg Com

What Does Dalton S Atom Look Like Quora

Dalton S Atomic Theory Overview Modern Application Expii
11 Which Of The Following Is The Major Drawback Of Dalton S Atomic Theory Atoms Of An Element Have Exactly Thesame Massatoms Are Indivisibleatoms Of Different Elements Havedifferent Massesall Of The Above Snapsolve

John Dalton Atomic Theory Discovery Experiments Biography

Solved Which Of The Following Statements Is False According Chegg Com

Solved Dalton S Atomic Theory Explained The Observation That The Percentage By Mass Of The Elements In 3 Compound Is Always The Same Thus Dalton S Atomic Theory Supports What Law The Law Of Multiple

Explain Dalton S Atomic Theory

Solved 1 One Key Point In Dalton S Atomic Theory Was Chegg Com

Solved Which Of The Following Statements Is Not Part Of Chegg Com

Solved Which Of The Following Reactions Is Possible Chegg Com

Atomic Structure Essential Question Describe How The Model Of The Atom Has Changed Since The Greek Idea Of Atomos Ppt Video Online Download

4 Atomic Structure Chemistry Batz

Lesson Explainer Modern Atomic Theory Nagwa

Dalton S Atomic Theory Chemtalk
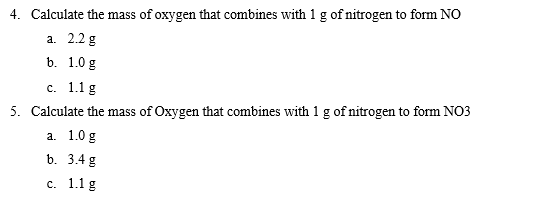
Solved 1 One Key Point In Dalton S Atomic Theory Was Chegg Com


Comments
Post a Comment